Actiphage TB uniquely detects active TB
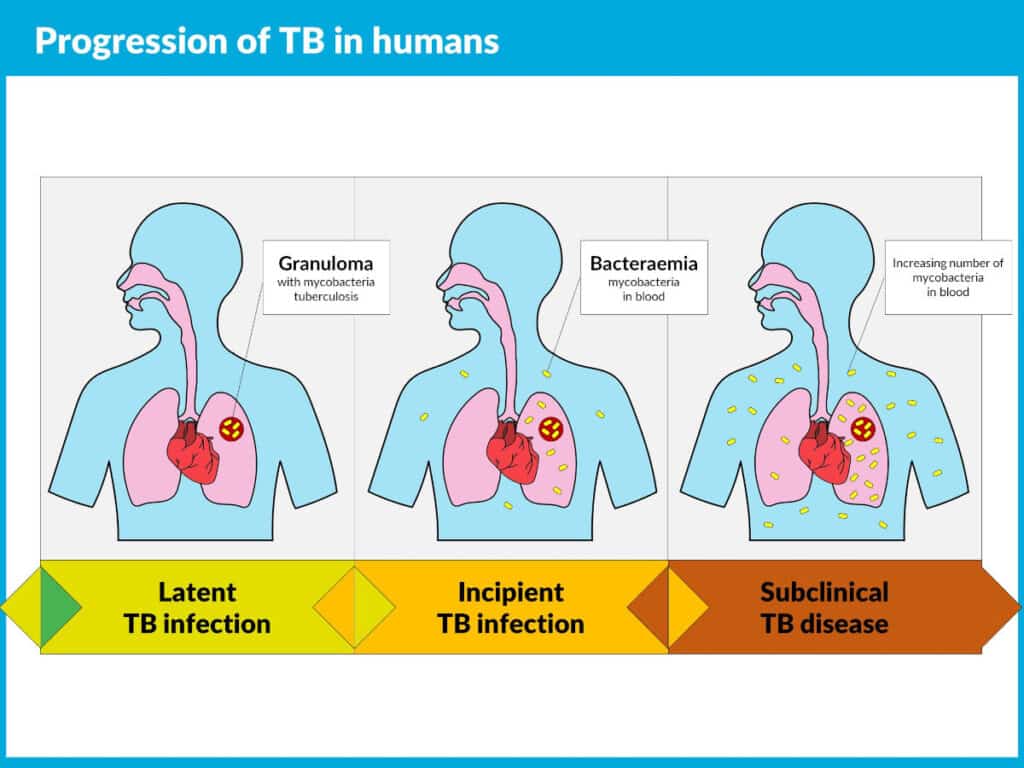
Tuberculosis is the world’s most fatal infectious disease, and it can lay dormant in an infected individual until their immune system is compromised by another infection, stress or malnutrition. The Actiphage TB test is able to detect the pathogen Mycobacterium tuberculosis (Mtb) in a blood sample when it escapes the body’s immune system, offering potential for a biomarker of disease progression.
Actiphage TB:
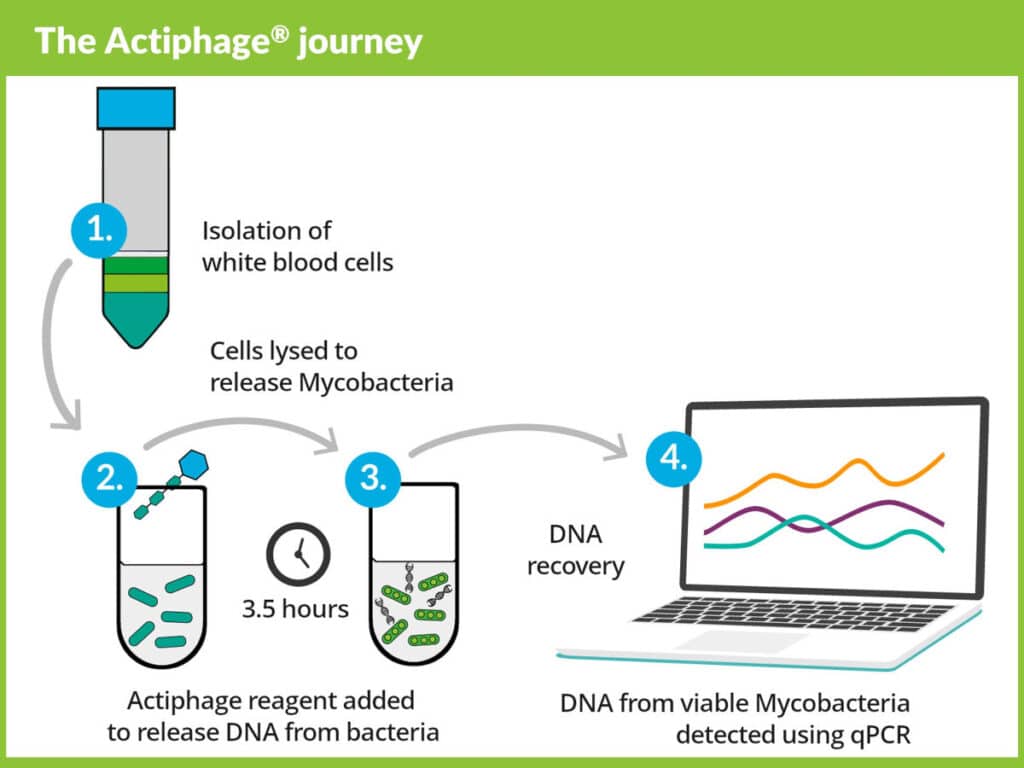
- the only blood-based test that combines phage technology, to detect only active, disease-causing mycobacteria, with traditional qPCR methods.
- leverages phage-based technology that is unique and detects only active TB bacterial cells, inherently leading to the higher specificity than other diagnostic methods.
- is a new, innovative TB diagnostic, is being developed as an incipient TB test and may allow for the scale-up of test-and-treat campaigns.
- may play roles in TB screening across the continuum of TB infection and management of TB drug therapy.
In the fight to End TB, Actiphage TB has potential to detect those with progressive disease. Also to monitor the efficacy of drug treatment – a test of cure – something that is not possible with current technology.
Actiphage has not received market clearance status by the U.S FDA or any other country’s regulatory authority.
Contact Us
General contact form