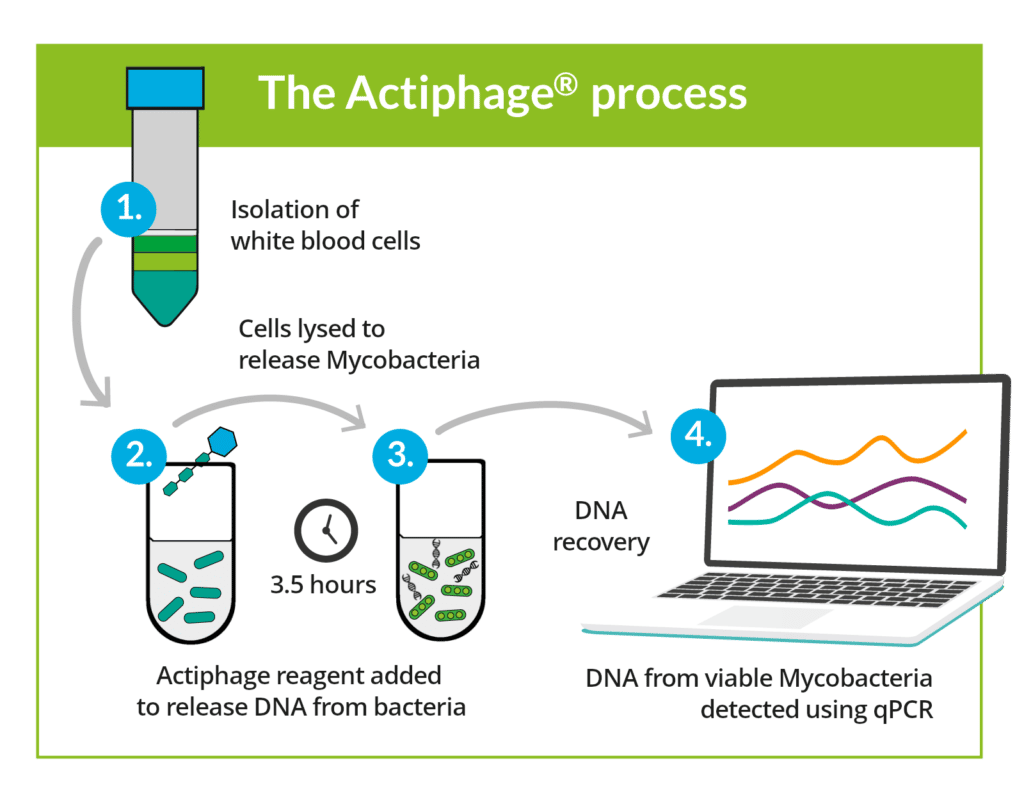
There are many benefits of Actiphage.
Rapid turnaround time
Actiphage only requires one visit to collect blood samples and analysis takes 6-8 hours to produce a result.
High sensitivity
The test only works on viable, active mycobacteria. Actiphage is more sensitive than any other test currently available, able to detect fewer than 10 live mycobacteria in a 2ml blood sample.
High specificity
The technology has built-in high-level specificity controls and is able to identify any species of mycobacteria that is susceptible to the bacteriophage and distinguish between them.
High accuracy
Actiphage can reproducibly distinguish between live and dead mycobacteria, meaning that a positive result will only be delivered if there are live mycobacteria in the sample.
Benefits of Actiphage
Dr Pranab Haldar, Clinical Senior Lecturer at the University of Leicester, UK, is based in a national TB hotspot. He has conducted several clinical trials with Actiphage and comments that it is a promising new type of biomarker as it can identify the organism itself in a blood sample and does not rely on the host immune response.
“The host immune biomarkers we currently use can tell us about the presence of current or previous TB infection. They cannot discriminate between the two or tell us anything about the infecting pathogen.
“Pathogen-directed biomarkers may allow us to discriminate between people who do and potentially don’t require treatment for their TB infection. This would enable targeting of treatment – that’s very powerful.”
Click here to contact us to find out more about the benefits of Actiphage.