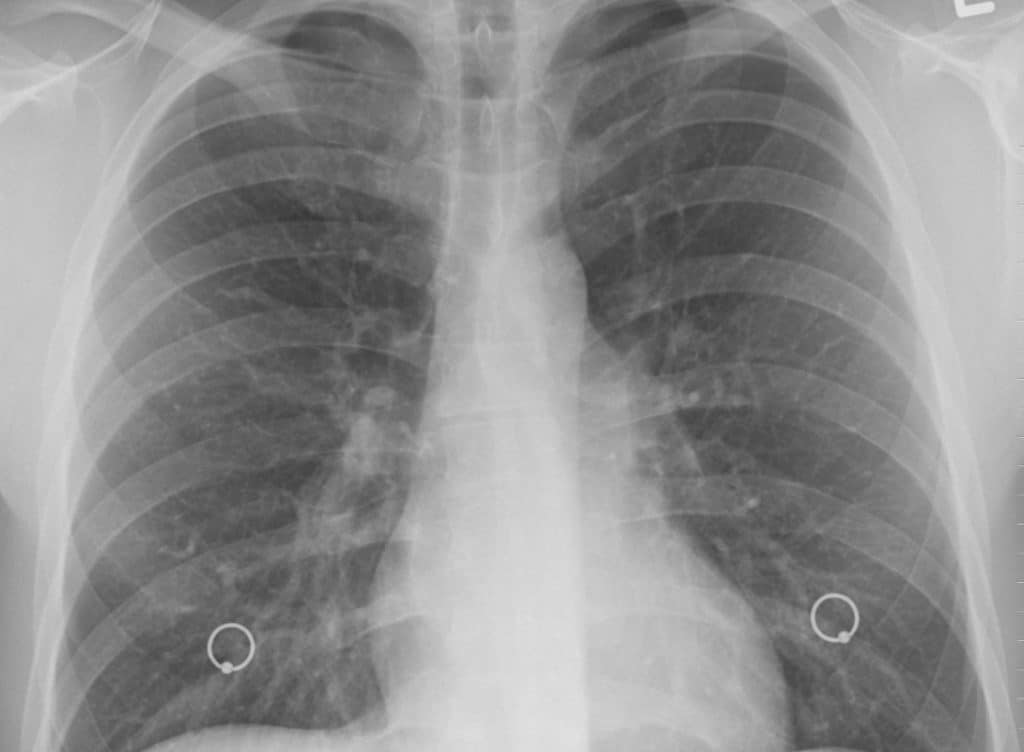
Actiphage®, developed by PBD Biotech, is a blood test for TB that was originally developed for cattle and other animals, which offers potential as an alternative diagnostic.
Following its successful proof-of-concept trial, the University Hospitals of Leicester have gained further funding for a pilot programme as the next step towards full validation of Actiphage for use in humans.
The collaborative study, between the University Hospitals of Leicester, University of Nottingham and PBD Biotech, has gained Medical Research Council funding. This funding has allowed a larger cohort of patients to be tested and researchers are co collecting data from almost 100 patients with both active and latent TB infection. The aim is to provide additional evidence for the use of Actiphage as a rapid, cost-effective tool to detect Mycobacterium tuberculosis, the pathogen that causes TB in humans.
TB is the world’s most deadly infectious disease and latest figures show an estimated 10 million people worldwide fall ill with the serious bacterial infection (WHO, 2018).
Currently, the only rapid diagnostics for TB rely on testing sputum – phlegm brought up from the deep in the lungs – however 40% of adults with TB and the majority of children struggle to bring up phlegm. Additionally, it is difficult to culture the slow growing bacteria on plates, yet this is the current gold standard diagnostic.
Actiphage can detect mycobacteria in a single blood sample. It uses a specific bacteriophage virus that multiplies rapidly in live mycobacterial cells; a natural process, which causes the cells to burst open releasing their DNA that can then be identified using PCR tests. So, Actiphage can be used to detect very low levels of infection, often before symptoms have developed and the disease becomes contagious.
The earlier proof-of-concept work using Actiphage, published in medical journal Clinical Infectious Diseases (June 2019), showed that the test could detect the presence of Mycobacterium tuberculosis in blood – not only in patients with active disease but also in the very early stages of infection.
The test was able to identify live mycobacteria in the blood of three patients with latent TB, and two of these were the only ones from the latent TB group who went on to developed active TB more than six months later; indicating that Actiphage could have a predictive role in identifying people with the infection at risk of developing the disease.
Mark Hammond, CEO of PBD Biotech, comments: “Actiphage is a unique blood test for TB and is currently being used to improve disease management within farm animals and wildlife, to provide early detection of disease. It is known that TB is zoonotic and can be transmitted from animals to humans, or through unpasteurised dairy products, so having a rapid tool that can be deployed in a One Health approach to managing the disease would be highly beneficial.”
Preliminary results and early evidence from the latest Actiphage trial across Leicester’s Hospitals will be available mid-2020.