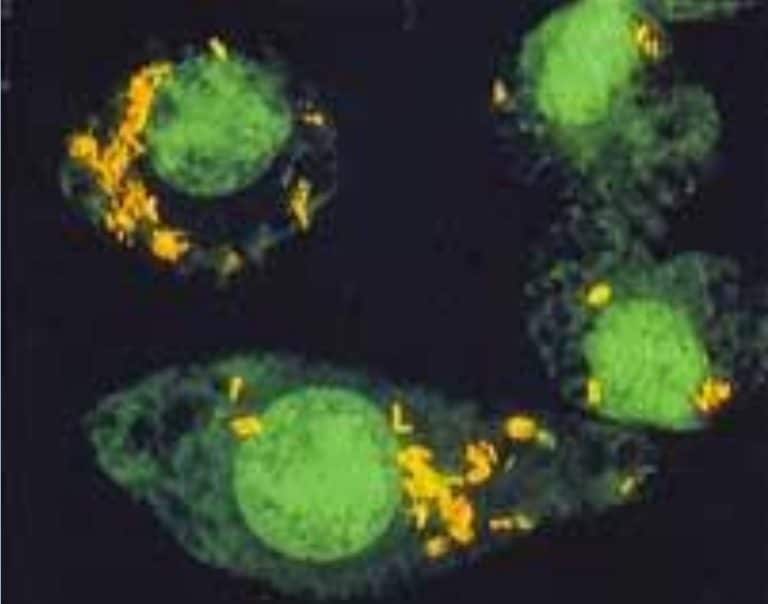
Actiphage® is phage-based technology that is able to detect extremely low levels of live mycobacteria in a wide range of species and samples.
The phage assay referred to in the following research papers is PBD Biotech’s patented Actiphage product.
Human TB
Proof of concept study uses high sensitivity Actiphage assay to identify low level Mycobacterium tuberculosis in immunocompetent patients with active and incipient TB:
Verma, R. et al, ‘A novel high sensitivity bacteriophage-based assay identifies low level M. tuberculosis bacteraemia in immunocompetent patients with active and incipient TB’, Clinical Infectious Diseases, ciz548
Jee Whang Kim et al, A novel bacteriophage-based assay stratifies tuberculosis risk in recent household contacts of pulmonary tuberculosis: A prospective observational cohort study
Presented at 32nd European Congress of Clinical Microbiology & Infectious Diseases April 2022
Further trials are underway in the UK, Canada and internationally. Please contact us for more information.
Johne’s Disease – cattle blood samples
The development and use of Actiphage® to detect a range of mycobacteria, including bovine TB and Johne’s disease, from blood samples [as mentioned above]:
B. M. C. Swift et al, ‘The development and use of Actiphage® to detect viable mycobacteria from bovine tuberculosis and Johne’s disease‐infected animals‘, Microbial Biotechnology (2019)
Detection of Johne’s disease infection of new-born calves in the first days of life:
Pelletier C. et al, ‘Detection of active infection of new-born calves by Mycobacterium avium subsp. paratuberculosis (MAP) in first days of life‘, Abstract presented at the European Association of Veterinary Laboratory Diagnosticians Congress (2018)
Improving rapid phage-based detection of Johne’s disease in the blood of experimentally infected cattle:
B. Swift et al, ‘Evaluation of the limitations and methods to improve rapid phage-based detection of viable Mycobacterium avium subsp. paratuberculosis in the blood of experimentally infected cattle‘, BMC Veterinary Research (2016), 12 (1), 115
Development and evaluation of a rapid phage-based method for detection of Johne’s disease in cattle blood:
Swift B. et al, ‘Development of a rapid phage-based method for detection of viable Mycobacterium avium subsp. paratuberculosis in blood in 48h‘, Journal of Microbiological Methods (2013), 94 (3), 175-179
Johne’s Disease – blood samples of other livestock
Development of a rapid method for detection of Johne’s disease in blood samples from farmed deer:
Kubala A, et al. ‘Development of a method to detect Mycobacterium paratuberculosis in the blood of farmed deer using Actiphage® Rapid‘, Frontiers in Veterinary Science (2021) 8:665697
MAP – Mycobacteria in dairy products
Survival of MAP in retail-bought pasteurised milk:
Gerrard Z.E. et al, ‘Survival of Mycobacterium avium subspecies paratuberculosis in retail pasteurised milk‘, Food Microbiology (2018), 74, 57-63
Detection of live MAP in powdered infant formula by phage assay:
Botsaris G. et al, ‘Detection of viable Mycobacterium avium subspecies paratuberculosis in powdered infant formula by phage-PCR and confirmed by culture‘, International Journal of Food Microbiology (2016), 216, 91-94
Bovine TB – cattle blood samples
The development and use of Actiphage® to detect a range of mycobacteria, including bovine TB and Johne’s disease, from blood samples:
B. M. C. Swift et al, ‘The development and use of Actiphage® to detect viable mycobacteria from bovine tuberculosis and Johne’s disease‐infected animals‘, Microbial Biotechnology (2019)
Gatcombe Farm – a novel enhanced cattle testing programme, overseen by vet Dick Sibley to achieve Officially TB Free status:
R. J. Sibley BVSc HonFRCVS, ‘The investigation of a persistent outbreak of bovine tuberculosis using a novel enhanced cattle testing programme and evaluation of environmental contamination‘, Paper presented at the British Veterinary Cattle Association Congress (2018)
Evidence of bovine tuberculosis in skin test positive cattle detected using 48 hour phage amplication testing protocol:
Convery T., Rees C. et al, ‘Evidence of Mycobacterium tuberculosis complex bacteraemia in intradermal skin test positive cattle detected using phage-RPA‘, Virulence (2016), 7 (7), 779-788
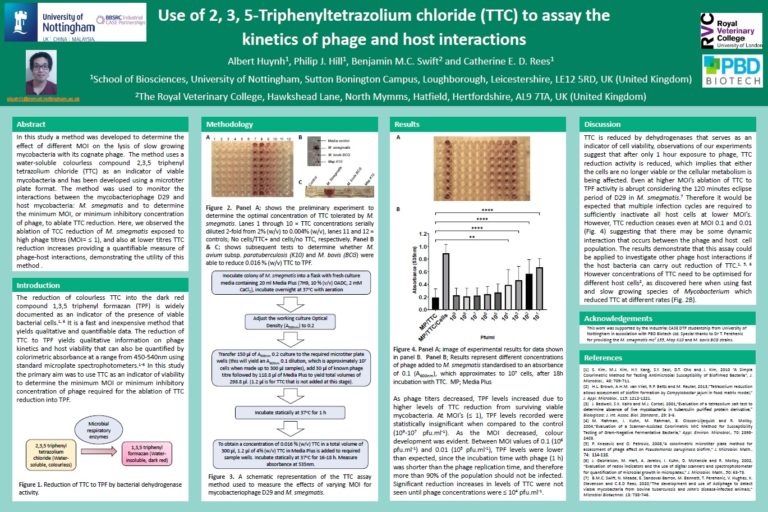
Multiplicity of infection:
Huynh A., Hill P., Swift B., Rees C, ‘Use of 2, 3, 5-Triphenyltetrazolium chloride (TTC) to assay the kinetics of phage and host interactions‘, Poster presented at Phages 2021
Developments in phage-based diagnostics
The Application of Bacteriophage Diagnostics for Bacterial Pathogens in the Agricultural Supply Chain: From Farm-to-Fork
Helen J. Jones, Christopher G. Shield, and Benjamin M.C. Swift
Published Online:16 Dec 2020 https://doi.org/10.1089/phage.2020.0042