Costed public health plan with high ROI aims to cut deaths by 90% in 8 years
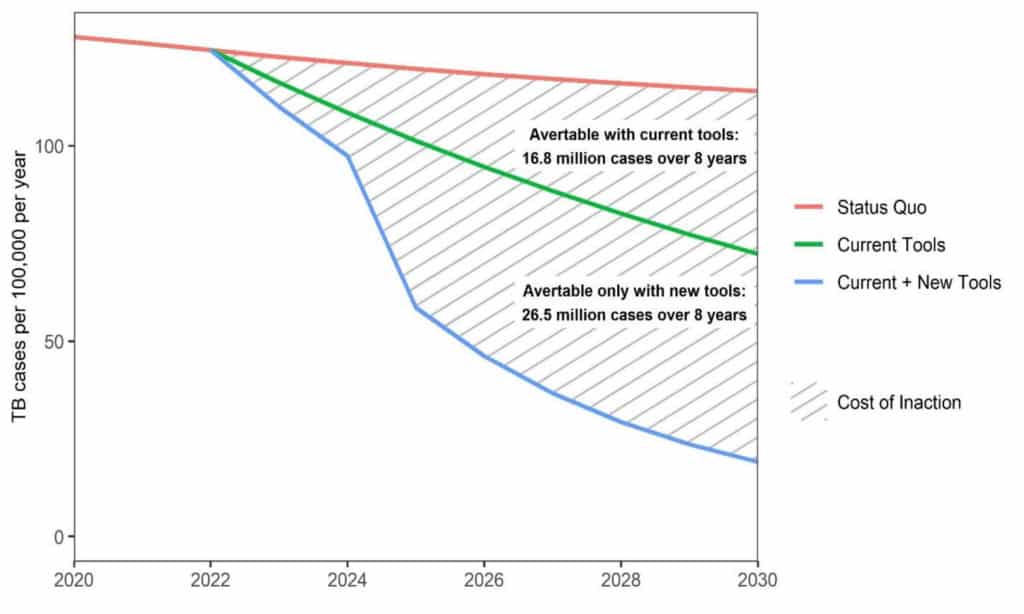
The Global Plan to End TB 2023-2030, launched on the 6th July 2022, is a detailed and costed action plan for ending TB as a public health challenge. It aims to cut 26.5 million cases in eight years. A key recommendation of the report is to universally replace sputum microscopy with rapid molecular diagnostics as the initial diagnostic test.
In 2020 it was estimated that nearly 10 million people had tuberculosis (TB), with deaths increased for the first time in over a decade. 4,100 people die each day from this treatable disease.
More than a third of people with TB in 2020 went undiagnosed, with each untreated case infecting 15 more
The Global Plan to End TB 2023–2030 is an inclusive document developed in collaboration with numerous partners, stakeholders and experts. It provides a clear roadmap and the most detailed budget estimates to date for ending TB as a public health challenge by 2030, in line with the UN Sustainable Development Goals.
The investment case for the estimated budget of US$249.98 billion required shows impressive returns on investment – US$40 for every dollar invested – and points out the huge human cost of inaction.
This Global Plan also re-imagines TB care to be focused on people and responsive to gender needs, taking into account the mental health challenges and the interplay with different diseases like HIV/AIDS. The plan offers robust guidance on the R&D investments needed to accelerate the development of new TB vaccines, diagnostics and medicines.
The report identifies that rapid and accessible TB diagnosis is the first step in providing effective treatment and saving lives, stating: “Many countries today still rely on sputum microscopy as the initial diagnostic test for TB. Rapid molecular tests need to replace sputum microscopy as the initial diagnostic test.”
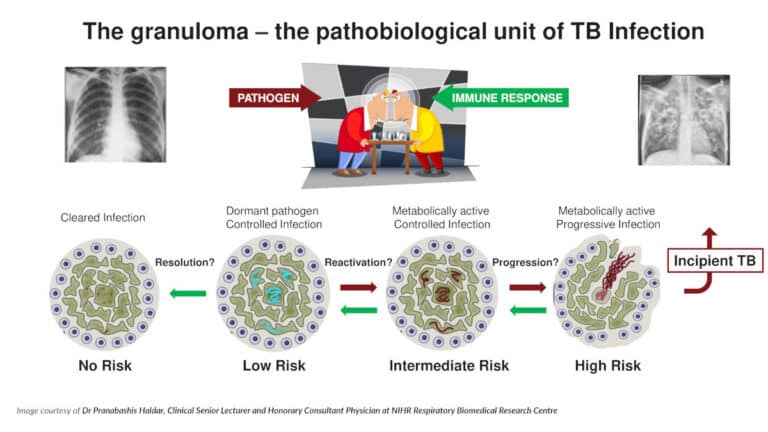
It also recognises the need to address the massive reservoir of TB infection, where there are no clinical symptoms, as these may eventually progress to active disease. It recognises that the current tuberculin skin test (TST) is insufficient as it can show a false-positive result in people who have received the Bacille Calmette-Guérin (BCG) vaccine.
The Global Plan to End TB makes the following recommendations for diagnostics:
- Develop rapid, affordable tests for diagnosis or triage that do not rely exclusively on sputum and are used at the point of care.
- Develop accurate drug susceptibility testing (DST) for critical medicines, including through sequencing-based tests and strategies for early detection of resistance to the medicines used in regimens.
- Improve tools for detecting TB infection (i.e. latent TB) and subclinical TB, and testing for risk of progression to active disease.
- Develop and harness AI and machine learning-based tests.
In conclusion, H.E. Mr Mansukh Mandaviya, Chair of the Stop TB Partnership Board, and Minister of Health and Family Welfare for the Government of India, says: “Beyond everything else, the Global Plan is about people and is dedicated to the millions of people who make daily efforts to help those affected by TB; it promises that ‘we will end TB together’.”
PBD Biotech, in collaboration with its partners, is making a contribution towards the report’s recommendations:
- Rapid affordable test for diagnosis or triage that does not rely on sputum.
- Improved tools for detection of latent TB and sub clinical TB.
- Clinical trials with vulnerable groups in countries with high rates of HIV/AIDS and TB.
The Global Plan to End TB 2023-2030 can be viewed here:
https://www.stoptb.org/global-plan-to-end-tb/global-plan-to-end-tb-2023-2030